Pharmacokinetics
Scroll down to learn about pharmacokinetics.
In this section you will learn about pharmacokinetics. You will encounter these concepts on school and national exams and on a daily basis at work, although you may not use those exact terms. Patients will ask questions about how their medications work, and the knowledgeable medical professional will be able to answer their questions with confidence.
Click through the toggles to learn about pharmacokinetics or skip down the page to play the games.
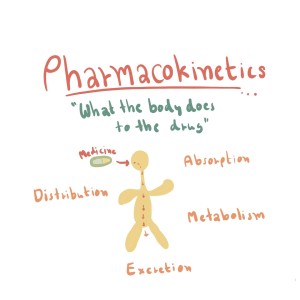
- Absorption: This process refers to how a drug moves from its site of administration into the bloodstream.
- Distribution: After a drug enters the bloodstream, it distributes throughout the body and reaches the target tissues or organs.
- Metabolism: Most drugs undergo chemical changes in the body, primarily in the liver, through various enzymatic reactions. These changes, known as biotransformations, can lead to the formation of active or inactive metabolites.
- Excretion: The final pharmacokinetic process is the elimination of the drug and its metabolites from the body, primarily through renal (kidney) or hepatic (liver) routes.
Pharmacokinetics may at first sound intimidating, but let’s bring it back down to Earth. Picture it as a fascinating journey of a medication inside our body, similar to the dramatic arc of a novel with four main stages: absorption, distribution, metabolism, and excretion. Let’s call these stages ADME for short, the ABCs of pharmacokinetics.
1. Absorption: Tickets, Please!
Imagine you’ve just taken a pill for a pesky headache. This pill, let’s call it our protagonist, starts its journey in your body via absorption. Absorption is all about how the drug moves from the outside world into your bloodstream. Think of it as the drug getting its ticket punched for the train ride at the station. Depending on the route of administration, this station could be your stomach, your intestines, or even your skin.
[s2If !is_user_logged_in() OR current_user_is(s2member_level0)]………..The full text is available to members.
[/s2If][s2If current_user_can(access_s2member_level1)]Now, not all drugs hop on the train at the same speed. Some get absorbed rapidly, while others take their time. Many factors can influence this, such as the drug’s formulation, the presence of food in your stomach, and the specific part of the body where the drug is introduced.
2. Distribution: The Grand Tour
Once our drug, now a full-fledged passenger in your bloodstream, it begins its grand tour of your body. This is called distribution. Your bloodstream is like the highway system of your body, carrying the drug to various “cities” or tissues.
Some drugs are attracted to busy, bustling cities like your heart or brain. Others prefer quieter places, like fat tissues. Drugs might also bind with proteins in the blood, essentially hitching a ride. How widely and quickly a drug is distributed depends on factors like blood flow, tissue type, and the drug’s ability to cross barriers like the blood-brain barrier, which acts like a strict bouncer at the entrance to the brain.
3. Metabolism: Makeovers and Modifications
But, just like in any epic tale, our protagonist undergoes some changes. This is metabolism. Primarily taking place in the liver, your body’s sophisticated “mechanic,” metabolism modifies the drug. It’s a bit like the drug going to a stylist for a makeover.
This makeover can make the drug more active (like a prodrug, which only works after metabolism), less active, or even inactive. Sometimes, it’s just about prepping the drug to be excreted, making it more water-soluble so it’s easier to remove. These changed versions of the drug are known as metabolites.
4. Excretion: Time to Say Goodbye
The final chapter in our drug’s journey is excretion. This is the body’s way of escorting the drug to the exit, primarily through the kidneys into the urine. Other ways of saying goodbye are through the lungs, sweat, and feces.
Excretion is crucial because it prevents the drug from overstaying its welcome. Too much of a good thing can be harmful, and that applies to medications too. The rate of excretion can impact how long a drug stays effective and can vary with factors such as age, kidney function, and hydration status.
Conclusion:
Pharmacokinetics, in essence, is the life story of a drug within our bodies. As medical professionals, understanding this story helps us grasp why we administer drugs the way we do, why certain drugs are not suitable for some patients, and why the timing of medication administration matters.
Remember, each drug is unique. They’re like characters in a novel, each with their own backstory and journey. So the more familiar you are with these journeys, the better you’ll be at predicting plot twists and turning them into better patient outcomes.
5. Factors Affecting Pharmacokinetics: The Plot Twists
Our drug’s journey isn’t always straightforward. Certain factors can create plot twists, altering how a drug is absorbed, distributed, metabolized, or excreted. Age is a big one: a drug may have a different adventure in an infant or elderly person compared to a young adult because of differences in organ function and body composition.
Health status also plays a role. Imagine if the highways (blood vessels) are under construction (due to a condition like atherosclerosis). The drug won’t be able to get around as quickly or easily.
Other plot twists might include genetic differences affecting how a person’s body handles a drug, interactions with other medications, and lifestyle factors like diet and exercise. Understanding these variables helps us predict how these stories might unfold differently in different people.
6. Application in Medical Practice: Using the Stories to Our Advantage
Here’s where pharmacokinetics leaps off the page and into your professional practice. Say you have a patient who needs pain relief but has poor kidney function. Knowing that certain pain medications are primarily excreted by the kidneys (our drug’s exit strategy), you’d know to consult with the healthcare team about adjusting the dose or possibly choosing a different medication to avoid potential harm.
Or perhaps you’re caring for a patient who is on a medication that’s metabolized in the liver (our drug’s makeover spot), but they also have liver disease. This knowledge alerts you to the need for careful monitoring for side effects or signs of toxicity, as the drug might hang around longer than usual.
Conclusion:
At its heart, pharmacokinetics is about stories – the epic adventures of drugs in the body. From their grand entrance (absorption), through their wide-ranging journeys (distribution), their striking transformations (metabolism), to their eventual departure (excretion), it’s a narrative filled with valuable insights.
By understanding these stories and their potential plot twists, you can apply this knowledge to improve patient care, reduce the risk of adverse drug reactions, and enhance therapeutic outcomes. Pharmacokinetics, then, isn’t just an intimidating term; it’s an invaluable tool in your professional toolkit.
[/s2If]Absorption is the first step in the pharmacokinetic process and refers to the passage of a drug from its site of administration into the systemic circulation (bloodstream). It is a crucial step that determines the onset, intensity, and duration of a drug’s action in the body.
- The rate of absorption determines how soon the drug will take affect.
- The amount of absorption determines the intensity of the drug’s effect.
………..The full text is available to members.
[/s2If] [s2If current_user_can(access_s2member_level1)]Factors influencing drug absorption include:
-
Drug formulation: The physical and chemical properties of a drug, such as solubility, particle size, and ionization state, can affect its absorption.
-
Route of administration: The route through which a drug is administered (e.g., oral, intravenous, intramuscular, subcutaneous, transdermal) can impact its absorption rate and bioavailability. See below for more information.
-
Physiological factors: Patient-specific factors, such as gastrointestinal motility, pH, and blood flow, can influence drug absorption.

Routes of Administration In pharmacology, the route of administration refers to the method used to introduce a drug into the body. The choice of administration route depends on factors such as the drug’s physicochemical properties, its intended effect, the desired onset and duration of action, and the patient’s age and medical condition. There are several routes of administration, which can be broadly classified into two categories: enteral and parenteral.
|
Distribution is the second step in the pharmacokinetic process, referring to the transport of a drug from the bloodstream to various tissues and organs throughout the body. Once a drug is absorbed into the systemic circulation, it can either remain in the bloodstream or distribute to the target and non-target tissues. The extent and rate of distribution are crucial in determining the drug’s therapeutic effects and potential side effects. Several factors can influence drug distribution:
[s2If !is_user_logged_in() OR current_user_is(s2member_level0)]………..The full text is available to members.
[/s2If] [s2If current_user_can(access_s2member_level1)]- Blood flow to tissues: The rate at which a drug reaches its target site depends on the blood flow to the specific tissue or organ. Organs with high blood flow, such as the liver, kidneys, and brain, receive drugs more rapidly, while those with lower blood flow, like adipose (fat) tissue, receive drugs more slowly.
- Plasma protein binding: Many drugs bind to plasma proteins, primarily albumin, in the bloodstream. Only the unbound (free) portion of the drug can distribute to tissues and exert its pharmacological effects. The extent of plasma protein binding can affect the distribution of a drug, as drugs with a high degree of protein binding have a limited ability to distribute into tissues. Very important concept! See more information below.
- Tissue permeability: The ability of a drug to cross biological membranes and enter cells or tissues depends on its physicochemical properties, such as lipophilicity (fat solubility) and molecular size. Lipophilic drugs can readily cross cell membranes, while hydrophilic (water-soluble) drugs have limited permeability.
- Tissue-specific factors: Certain tissues may have a higher affinity for specific drugs due to the presence of receptors or other binding sites. For example, some drugs have a high affinity for adipose tissue, which can serve as a reservoir for lipophilic drugs, prolonging their action and affecting their distribution.
|
More on Plasma Protein Binding Albumin is the most abundant protein in human plasma, making up approximately 50-60% of the total plasma protein content. One of its primary functions is to transport various molecules, including drugs, throughout the body. Drug-albumin binding occurs when a drug molecule forms a reversible bond with specific binding sites on the albumin protein. The binding affinity between a drug and albumin depends on various factors, such as the drug’s chemical structure, charge, and lipophilicity. It can also be influenced by the presence of other molecules competing for the same binding sites. The portion of the drug bound to albumin is considered pharmacologically inactive because it cannot cross cell membranes or exert its therapeutic effects. Only the unbound or free fraction of the drug can cross cell membranes and interact with its target receptors or enzymes and produce the desired therapeutic effects. The binding of drugs to albumin is significant for several reasons:
|
Metabolism is the third step in the pharmacokinetic process, which involves the biotransformation of a drug into different chemical compounds, known as metabolites. The primary goal of drug metabolism is to convert lipophilic (fat-soluble) drugs into more polar (water-soluble) metabolites, making them easier to excrete from the body. Metabolism can also result in the activation or inactivation of a drug, impacting its therapeutic effects and potential side effects. The liver is the primary organ responsible for drug metabolism, although other tissues, such as the gastrointestinal tract, kidneys, and lungs, may also contribute.
[s2If !is_user_logged_in() OR current_user_is(s2member_level0)]………..The full text is available to members.

|
Important Concept! First-pass Effect refers to the process by which a drug’s concentration is significantly reduced before it reaches the systemic circulation. This occurs when a drug is administered orally and absorbed from the gastrointestinal tract.
|
Drug metabolism in the liver is primarily mediated by enzymes, with the most significant enzyme family involved in this process being the cytochrome P450 (CYP450) enzymes.
There are more than 50 human CYP450 enzymes, which are divided into families and subfamilies based on their amino acid sequence similarities. The most clinically significant CYP450 families involved in drug metabolism are CYP1, CYP2, and CYP3.
Inhibition and Induction
In the context of drug metabolism, inhibition and induction refer to the processes by which a drug or other substance affects the activity of drug-metabolizing enzymes, particularly the cytochrome P450 (CYP450) enzymes. Both inhibition and induction can have significant clinical implications, as they can influence the rate at which a drug is metabolized, potentially leading to altered drug efficacy, side effects, and drug interactions.
- Inhibition: Inhibition occurs when a drug or other substance decreases the activity of a drug-metabolizing enzyme. This can result in a reduced rate of drug metabolism, leading to increased plasma concentrations of the affected drug. As a consequence, the risk of side effects or toxicity may increase.
- Induction: Induction refers to the process by which a drug or other substance increases the activity or expression of drug-metabolizing enzymes. Induction can lead to increased drug metabolism, resulting in reduced plasma concentrations of the affected drug, which may decrease its efficacy.
Some factors that can influence drug metabolism include:
-
Genetic variations: Individual genetic differences in the expression or function of drug-metabolizing enzymes can impact the rate and extent of drug metabolism, leading to variations in drug response and susceptibility to side effects.
-
Liver function: The liver’s metabolic capacity can be affected by factors such as age, disease, and nutritional status, which can alter drug metabolism and influence drug response.
-
Drug-drug interactions: The concomitant administration of multiple drugs can lead to interactions that affect drug metabolism, either by inducing or inhibiting the activity of drug-metabolizing enzymes.
-
Environmental factors: Lifestyle factors such as diet, smoking, and alcohol consumption can influence the expression and activity of drug-metabolizing enzymes.
Excretion is the fourth and final step in the pharmacokinetic process, which involves the elimination of drugs and their metabolites from the body. The primary goal of excretion is to remove these substances to prevent their accumulation and maintain a balance within the body. Efficient excretion is essential for terminating a drug’s action and preventing potential toxicity. There are several routes and mechanisms of excretion, with the kidneys and liver being the most important organs involved.
[s2If !is_user_logged_in() OR current_user_is(s2member_level0)]………..The full text is available to members.

- Renal excretion: The kidneys play a central role in the elimination of drugs and their metabolites, primarily through a process called glomerular filtration. In this process, blood is filtered through the glomerulus, a network of capillaries in the kidneys, and the filtrate containing water, electrolytes, and small molecules, including drugs and their metabolites, is collected in the renal tubules. Some substances in the filtrate are reabsorbed back into the bloodstream, while others are actively secreted into the tubules. The final filtrate, containing the drug and its metabolites, is excreted as urine.
-
Hepatic excretion: The liver is another essential organ involved in drug excretion, primarily through the process of biliary excretion. In this process, drugs and their metabolites are secreted into bile, which is then transported to the small intestine and eliminated from the body through feces. Some drugs and metabolites can be reabsorbed from the intestine back into the bloodstream, a process known as enterohepatic recirculation, which can prolong the drug’s action and affect its excretion.
-
Other routes of excretion include:
-
Pulmonary excretion: Some volatile drugs, such as anesthetic gases, can be eliminated through exhalation from the lungs.
-
Sweat, saliva, and tears: Small amounts of drugs and their metabolites can be excreted in sweat, saliva, and tears, although these routes are generally not significant for most drugs.
-
Breast milk: Some drugs can be excreted in breast milk, posing a risk to nursing infants.
-
Steps to Renal Drug Excretion
-
Glomerular filtration: The first step in renal excretion is the filtration of blood in the glomerulus, which is a network of tiny blood vessels (capillaries) within the kidney. Blood flows into the glomerulus, where hydrostatic pressure forces water, electrolytes, small molecules (including drugs), and waste products across the glomerular filtration barrier into the Bowman’s capsule. This filtrate, now called the glomerular filtrate, is essentially free of large molecules, such as proteins and blood cells.
-
Tubular secretion: After glomerular filtration, the filtrate enters the renal tubules. In this step, the tubular cells secrete certain substances, including drugs and their metabolites, from the blood into the tubular fluid. This process is particularly important for drugs that are not easily filtered through the glomerulus due to their size, charge, or protein binding. Active transport mechanisms, such as organic anion and cation transporters, are involved in tubular secretion, and they help move substances against their concentration gradients.
-
Tubular reabsorption: As the tubular fluid moves along the renal tubules (from the proximal tubule to the distal tubule and collecting duct), various substances, including water, electrolytes, and some drugs, are reabsorbed back into the bloodstream. The extent of reabsorption depends on the drug’s physicochemical properties, such as its lipophilicity (ability to dissolve in fat), ionization state, and molecular size. Reabsorption can occur through passive diffusion, facilitated diffusion, or active transport.
- Lipophilic (fat-soluble) drugs can easily cross cell membranes, including those lining the renal tubules. As the tubular fluid moves along the nephron, lipophilic drugs may passively diffuse back into the bloodstream due to their ability to dissolve in the lipid bilayers of the tubular cells.
- It is for this reason that the liver strives to metabolize lipophilic drugs into a more polar form (hydrophilic), to aid in excretion of drugs from the body.
- Lipophilic (fat-soluble) drugs can easily cross cell membranes, including those lining the renal tubules. As the tubular fluid moves along the nephron, lipophilic drugs may passively diffuse back into the bloodstream due to their ability to dissolve in the lipid bilayers of the tubular cells.
Terms to Know – Pharmacokinetics
Pharmacokinetics also refers to the movement of a drug throughout the body.
Each phase of pharmacokinetics – absorption, distribution, metabolism, and excretion – involve drug movement through membranes. Drugs pass through membranes in a variety of ways but three are most important – Direct penetration, facilitated diffusion, and active transport.
- Direct penetration is the process by which drugs cross biological membranes, such as the cell membrane’s phospholipid bilayer, by passive diffusion without the need for membrane transporters, channels, or the expenditure of energy. This is the most common method of drug transport as most drugs are too large to fit through channels and pores and most lack a transport system.
Drugs that are lipid soluble are absorbed more rapidly because they can readily cross cell membranes.
Cell membranes are composed of bilayers of lipids (fats) and phosphate “tails.” A common rule of chemistry is that “like dissolves like.” Because cell membranes are composed of lipids, a drug must be lipid soluble (lipophilic – “lipid loving”) to directly pass through a cell membrane. Some drugs are not lipophilic, rather they are hydrophilic (water loving) and cannot pass through cell membranes via direct penetration. Think about mixing water and oil. You can shake ajar of the two, but they will never dissolve into each other. They separate and settle into layers.
-
Facilitated Diffusion – This mechanism involves the passive movement of drug molecules across membranes with the help of specific carrier proteins. Facilitated diffusion does not require energy expenditure but is limited by the availability of carrier proteins and can become saturated at high drug concentrations.
-
Active Transport – In this process, drug molecules are transported across membranes against their concentration gradient with the help of carrier proteins and energy derived from ATP (adenosine triphosphate). This mechanism allows the absorption of drugs that are not easily absorbed by passive diffusion or are too large to pass through membrane pores.
Minimum Effective Concentration (MEC) refers to the lowest concentration of a drug in the bloodstream that is required to produce a desired therapeutic effect.
If the drug concentration falls below the MEC, the therapeutic effect may be insufficient or lost, leading to suboptimal treatment outcomes.
Toxic concentration refers to the level of a drug or substance in the bloodstream at which it causes harmful or adverse effects on the body. When the concentration of a drug exceeds the maximum safe concentration (MSC) and enters the toxic range, it can lead to undesirable side effects, organ damage, or even life-threatening situations.

A higher therapeutic index indicates a wider margin of safety, as there is a greater separation between the effective dose and the toxic dose. In other words, a drug with a high TI is considered safer because there is a lower risk of toxic effects at therapeutic doses.
Conversely, a drug with a low therapeutic index has a narrow margin of safety, meaning that even small changes in the dosing regimen or individual patient factors could lead to toxic effects.
Trough level – Also known as the minimum concentration, refers to the lowest concentration of a drug in the bloodstream, typically measured just before the administration of the next dose.
It is commonly used in therapeutic drug monitoring to ensure that drug concentrations remain within the therapeutic range, which is the range of drug concentrations associated with optimal efficacy and minimal side effects.
Monitoring trough levels is particularly important for drugs with a narrow therapeutic index, where small changes in drug concentrations can lead to significant differences in therapeutic effects or toxicity.
Eexamples of such drugs include
- vancomycin
- lithium
- digoxin
- warfarin
The half-life of a drug is a pharmacokinetic parameter that represents the time it takes for the concentration of the drug in the body to decrease by half. It helps healthcare professionals determine the appropriate dosing frequency and duration of treatment.
Half-life is influenced by two main processes: absorption and elimination. Absorption refers to how a drug enters the bloodstream, while elimination refers to the removal of the drug from the body, either through metabolism or excretion.
Several factors can affect a drug’s half-life, including:
-
Drug formulation: The formulation of a drug, such as immediate-release or extended-release tablets, can impact its absorption rate and thus its half-life.
-
Route of administration: The way a drug is administered (e.g., oral, intravenous, intramuscular) can affect its absorption and elimination, impacting the half-life.
-
Metabolism: The rate at which a drug is metabolized in the body can influence its half-life. This rate can vary among individuals due to factors such as genetics, age, and co-existing medical conditions.
-
Elimination: The efficiency of the body’s organs, such as the liver and kidneys, in eliminating a drug can affect its half-life. Factors like renal or hepatic impairment can significantly extend drug half-life.
Drugs with a short half-life may need to be administered more frequently to maintain their therapeutic effect, while those with a long half-life may require less frequent dosing.
Plateau drug levels, also known as steady-state concentrations, refer to the point at which the rate of drug administration (input) is equal to the rate of drug elimination (output) in the body.
At this stage, the drug concentration in the bloodstream remains relatively constant, fluctuating within a narrow range around an average value. Achieving plateau drug levels is crucial for maintaining consistent therapeutic effects during the course of treatment.
After repeated and regular administration of a drug, it typically takes about 4-5 half-lives for the drug to reach steady-state concentrations. The half-life of a drug is the time it takes for the drug’s concentration in the body to decrease by 50%. For drugs with short half-lives, plateau levels are reached relatively quickly, while drugs with long half-lives may take days or even weeks to reach steady-state concentrations.
In some cases, therapeutic drug monitoring may be employed to measure drug concentrations in the patient’s blood, allowing for adjustments in dosing to maintain plateau levels within the desired therapeutic range.
A loading dose is an initial, higher dose of a drug administered at the beginning of treatment to rapidly achieve the desired therapeutic concentration in the bloodstream.
This practice is particularly useful for drugs with long half-lives, as it can take a significant amount of time to reach steady-state concentrations with regular dosing alone. The loading dose helps to quickly establish an effective drug concentration, allowing the desired therapeutic effect to be achieved sooner.
After administering the loading dose, maintenance doses are given at regular intervals to maintain the therapeutic drug concentrations within the desired range.
Course and Games to Test Your Knowledge
Click on the corresponding image below to test your knowledge of pharmacokinetics. The mini course allows you to choose between flashcards, a randomized quiz, as well as line-’em-up and drag-and-drop interactions. The other options are games with rationales.
|
Pharmacokinetics Mini Course [s2If !is_user_logged_in() OR current_user_is(s2member_level0)] The course is available to members. [/s2If] [s2If current_user_can(access_s2member_level1)] [/s2If]
|
|
|
Pharmacokinetics 1 [s2If !is_user_logged_in() OR current_user_is(s2member_level0)]Printouts available to members [/s2If]
[/s2If] |
Pharmacokinetics 2 [s2If !is_user_logged_in() OR current_user_is(s2member_level0)]Available to members [/s2If]
[/s2If] |







